The brain is a complex system in which processing occurs at different scales in an interconnected architecture involving a large number of interacting variables. There is a growing conviction that deeper knowledge of brain function passes through the understanding of such structural architecture. For this reason, increasing effort has been put on the investigation of both the structural and functional connectomes, i.e., the whole-brain reconstruction of the neuronal structure and function at multiple scales: from the micro-scale (one or few cells) to the macro-scale (the whole brain ).
Reconstructing the brain connectome across these scales is fundamental to understanding the constituent parts of the nervous system, their multiple interactions and the advanced cognitive functions that they support. This can provide novel insights into the mechanisms that generate abnormalities and pathologies. However, still, there is a lack of tools allowing to fuse the data produced at multiple scales, and connectomic studies are still hindered by the limits of the current tools. Moreover, beyond the mutli-scale issue, functional and structural connectivity analyses are often conducted independently, considering one modality as complementary to the other. Nevertheless, the function is modulated by the structure and there is an increasing need for a more integrated multimodal analysis allowing to understand how the structural and functional connectivity relate to one another.
To this aim, it becomes fundamental to capture how neurons or brain areas collectively work to accomplish the basic tasks on top of which high-level functions are built. The complexity of such interactions, generates new requirements that can be addressed by computational tools rooted in multimodal analysis methods. Our aim is, therefore, to develop novel computational approaches allowing to investigate the brain connectome at different scales, operating on both functional and structural information and eventually integrating them in an "effective connectome". The plan is to generate tools grounded on machine learning and pattern recognition methods allowing to investigate which elements in the connectivity characterize the different pathologies.
Analysis of brain networks from neuroimaging data: macro-scale connectomicsNeuroimaging technologies (e.g., MRI, EEG, MEG, etc.) provide an abundance of multimodal information on brain connectivity, whose interpretation requires advanced computational approaches. Classical techniques based on graph theory have been extensively applied to investigate overall connectivity from neuroimaging data in terms of connectivity indices. However, the interpretation of these indices in biological terms remains elusive due to the unknown biological underpinnings of the connectivity patterns. Our aim is therefore to analyze such connectivity patterns and how their variability allows to explain and characterize pathologies and dysfunctions in the brain. Case-control characterization through supervised classificationIdentifying imaging biomarkers characterizing the presence or the absence of a disease is an important step toward improving our understanding of the biological basis of mental health (autism, schizophrenia) and neurodegenerative disorders (Alzheimer's, Parkinson's, multiple sclerosis) alike. The aim of this research activity is to exploit machine learning methods to find the elements in the connectomes that characterize the differences between case and control groups. 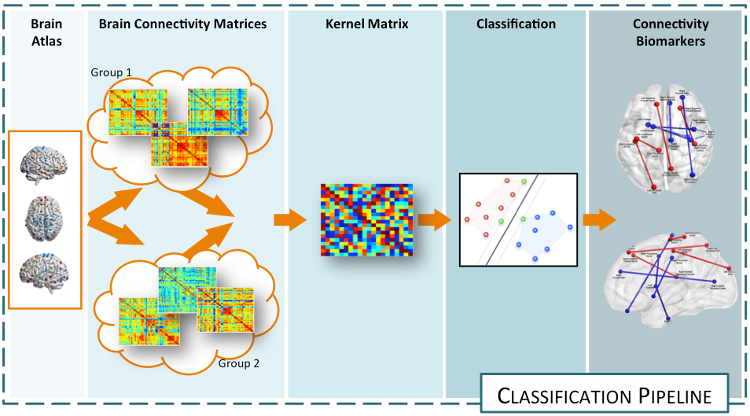 The idea is to solve a classification task whose side-effect is an identification of the features that better describe the differences between groups of subjects. The main issue to address in this problem is the data representation. Indeed, this task need to be solved working directly on the connectomes, hence, graph representation plays an important role. In this framework, we are developing various methods exploiting various technologies (e.g., sparse learning, graph Laplacians, manifold learning, etc.) allowing to discriminate healthy from pathological subjects, characterizing the abnormal connectivity between brain regions. Developped approahes can be applied to any connecome, both functional and structural. References:
|
White matter fiber segmentation and connectivity-based atlasesDiffusion MRI allows neuroscientists to study the anatomical organization of the brain and surgeons to plan their interventions. To this aim a number of algorithms have been designed to compute the white matter tractography, i.e., the set of fibers describing the brain macro connectivity. The set of tracts from whole-brain tractography, however, contains an overwhelming amount of information and neuroscientists need tools allowing to retrieve white matter fiber bundles of potential interest. Without a-priori hypotheses on the bundles to extract, the simplest strategy is to resort to unsupervised learning algorithms. 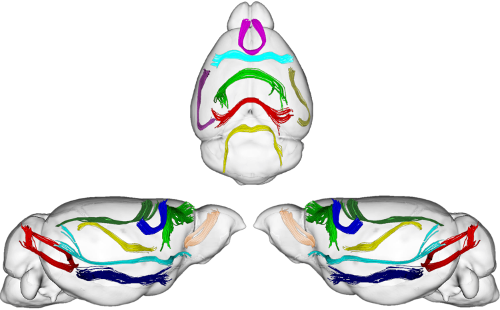 Consequently, we are working with a multi-subject white matter fiber clustering method, based on a graph theory approach known as Dominant Set clustering. This method has some advantages that make it attractive for this task, and through its use, we are able to segment white matter fibers into bundles. Beyond allowing the neuroscientists to easily identify the main physical connections in a single subject or at the population level, this information can be used as the starting point for further research activity related to brain cortex parcellation based on white matter structure. References:
|
Multi-Modal Community DetectionThe advent of functional connectomics as a mean to probe the brain mechanisms is a fundamental change from previous approaches. As a result, it is now common practice to exploit graph theory tools to characterize brain functions with a graph-based perspective which was not used before. In particular, one of the endeavor in connectomics is to detect the community structure of functional networks, typically determined from resting state fMRI. In this framework, our research activity aims at investigating alternative machine learning approaches for retrieving functional and structural communities shared across multiple-subjects, i.e. relying on unsupervised machine learning methods. 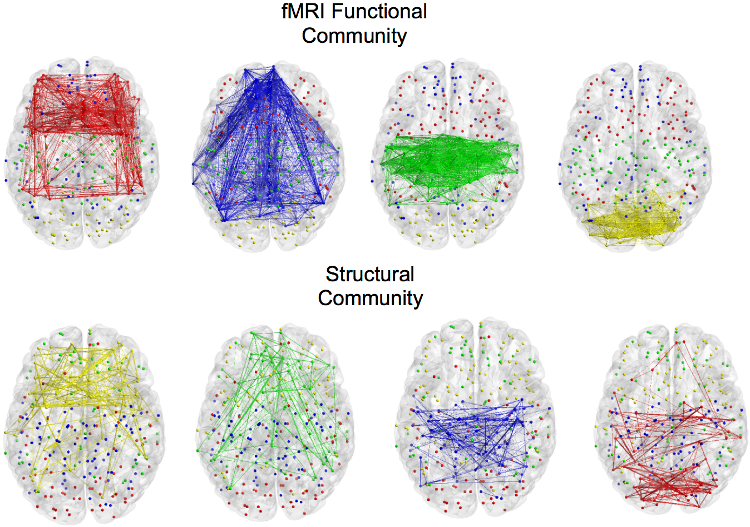 Group-wise characterization of brain connectivity usually passes through the construction of an average graph. However, this might introduce artifacts with connections presenting high cross-subjects variability. To cope with this issue, we introduced an approach based on the joint diagonalization of multiple graph Laplacians. With this approach, we built a joint eigenspace where most important eigenvectors can be used to compute a group-wise spectral clustering. This allows to find a brain sub-network organization across subjects but also across modalities, mixing structural and functional connectivity. References:
|
Dynamical Functional ConnectivityA recent trend in the analysis of functional connectivity determined from resting state fMRI is to process it as a dynamic system, where the connectivity changes in time. This novel view opens new challenges, such as how to model and interpret resting state networks changing over the time. Aiming at untangling dynamic functional connectivity (dFC), we developed a novel approach to extract traces of brain function by projecting dFC into a low-dimensional space, where the interpretation of dynamics becomes easier to recognize. As a testbed, we used fMRI data based on a movie-watching task, which allows us to evoke during fMRI acquisition different brain states reflecting the emotions felt by the subjects. In this way, it is possible to recognize different resting states conditions affected by the corresponding task. 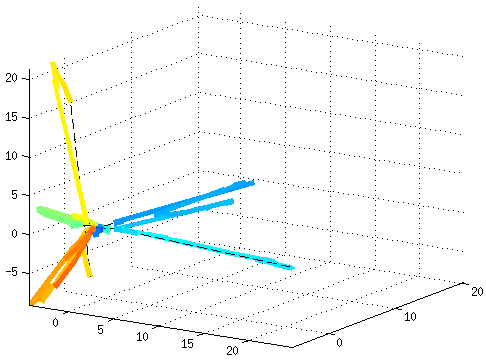 References:
|
Neuronal networks in fluorescence imaging: meso-scale connectomicsRecent improvements in multi-electrode arrays (MEAs) technology have provided new ways of collecting high-resolution structural and functional information at the scale of cellular networks. This allows to investigate both the microscale cellular features and how they aggregate at mesoscale when characterizing the connectivity patterns of neuronal networks. In other words, this new perspective provides the unique advantage of capturing fine neuronal interactions at network level while preserving the cellular resolution. 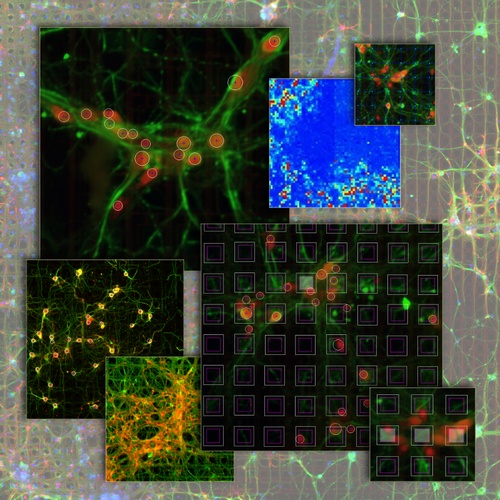 The research activity in this project aims at investigating meso-scale connectomics applied to multimodal MEA datasets, introducing novel computational methods for structural connectivity estimation and structure-function integration. Indeed, the final aim is to provide computational methods for a joint structural and functional analysis of neuronal networks, by integrating multimodal recordings. 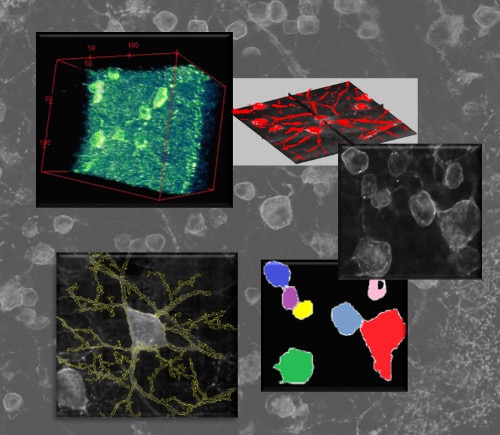 More specifically, our datasets consist of fluorescence microscopy images of neuronal cultures or tissues (e.g., retina), and electrophysiological signals of the neuronal functional activity, acquired with MEAs. The complexity of such data modalities requires advanced approaches for the analysis of both neurons and network morphology. Particularly, the computational methods we have been focusing on mainly involve: image processing and segmentation algorithms for the detection of neuronal nuclei and of tubular dendritic structures; probabilistic models for the reconstruction of neuronal connectivity and, more generally, advanced pattern recognition techniques. References:
|